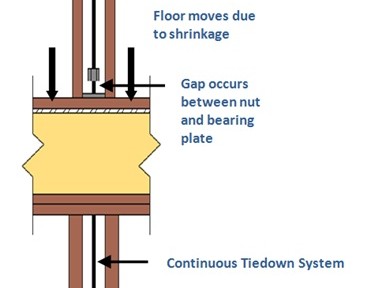
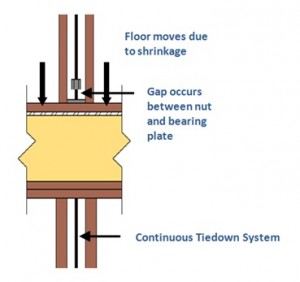
When you hear $452 billion, what comes to mind? Perhaps the annual state budgets of California, Texas, Florida or New York? Maybe the combined net worth of Bill Gates, Larry Ellison, or the Walton Family? While those would be good guesses, I bet you didn’t think of corrosion! According to a May 2012 Congressional Briefing hosted by NACE International and ASM International, corrosion-related costs are a staggering 3.1% of the U.S. GDP, which is more than the individual budgets of those states above, and the combined net worth of the top 15 people listed on the Forbes 400: The Richest People in America.
Corrosion of metallic surfaces is an electrochemical process typically involving an anode, electrolyte and a cathode. An anode is a metal zone which loses electrons when exposed to an electrolyte, an electrolyte is a non-metal electrical conductor, and a cathode is the zone where an oxidizing agent (e.g., oxygen) gains the electrons. While there are many different forms of corrosion (e.g., pitting, intergranular, wet storage stain, etc.), and various sources of causes (e.g., treated lumber, moisture level, temperature, atmosphere, air quality, etc.), other factors such as exposure related to time of wetness are equally important. In a study presented in Dr. X.G. Zhang’s book Corrosion and Electrochemistry of Zinc, time of wetness is 50% greater near the top of a structure compared to the bottom, leading to greater corrosion.