This week we are blogging about being “galvanic,” and we don’t mean with respect to people, but with respect to the corrosion that occurs between dissimilar metals.
Here is a question, and it is not a joke: What is one significant result that can occur when you have both electrochemical activity and intimate contact? The answer is galvanic corrosion.
Galvanic corrosion can take place when two or more metals of different electrochemical activity are in intimate contact in the presence of an electrolyte. The dissimilar metals form a galvanic couple, and with the aid of the electrolyte, a galvanic current flows between the metals of the galvanic couple. The more anodic metal corrodes in the presence of the more cathodic metal. In fastening systems, this can be a significant issue because the metal of the fastener often does not match that of the connection materials, making their electrochemical activity dissimilar.
Let’s examine the requirements for galvanic corrosion to occur.
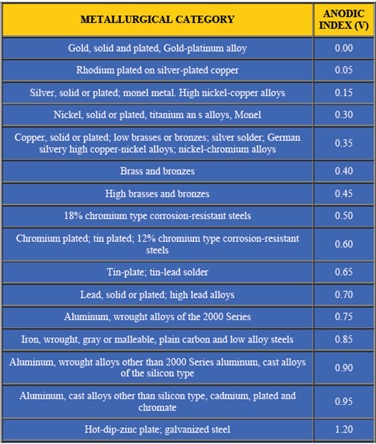
First – In the most common instance, the metals are dissimilar, which means that the metals have different chemical potentials. You may be familiar with the galvanic series where metals are rated by their tendency to give up electrons in a salt-water solution. See Figure 1 for a chart of the galvanic series. The chart is structured with the most cathodic metals at the top and progresses to the most anodic at the bottom. The anodic index shown in the chart is normalized so that gold is the minimum numerical value, while zinc has the greatest numerical value. Stainless steel (300 series) is hidden in the terminology of “18% Chromium type corrosion-resistant steels.” In this chart, the stainless steel is assumed to be passivated.
Second – The metals must be in direct contact.
Third – An electrolyte must be present to facilitate the movement of electrons. The electrolyte in construction environments is usually plain water that occurs in the form of precipitation, condensation or water splash. Electrolytes that are solutions of chlorides (for example, salt water) are particularly effective electrolytes because they are more conductive.
The size of the anodic and cathodic parts can also be important in galvanic corrosion. If the anodic area is small relative to the cathodic exposed area, then the severity of the anodic corrosion is amplified. We can write an equation to explain the role of area in the galvanic process. We know that no corrosion will occur if the corrosion current density (icor) in μA/cm2 is the same for the anode (icor-a) and the cathode (icor-c). Here we are using a and c as subscripts to identify the anode and the cathode in the galvanic system. We know that current density is a function of total anodic current (I) in μA (where italicized A is amps), and the exposed area (A) is in cm2, which (according to ASTM G102-89) can be written as
icor = Icor/A
No galvanic corrosion transpires if icor-a for the anodic material is equivalent to icor-c of the cathodic material, which is to say Icor/Aa = Icor/Ac. However, when Ac ≠ Aa, then the corrosion function is not balanced, and relative areas can drive the severity of the galvanic reaction. Inasmuch as area can affect the galvanic process, it will help connection performance if the more anodic material is larger than the more cathodic material. And, by making the Aa>>Ac, we can arrest or minimize the galvanic process. Generally, this means it is best to have a fastener that is more cathodic than the materials being fastened.
We also know that the environment can affect galvanic activity. The differential in the anodic index of dissimilar metals is amplified in harsh environments, but in controlled environments, a greater differential in anodic index can be tolerated.
Let’s summarize some best fastening practices for preventing galvanic conditions that could undermine an otherwise good connection design (Claus, L. 2014. “Galvanic Corrosion.” Fastener Technology, April, pp. 64–66.):
- Use fasteners that are galvanically similar to the connection materials.
- Isolate the dissimilar materials by using a plastic washer or durable coating.
- Prevent entrapment of water or shield the connection from direct weather exposure.
- If the fasteners are dissimilar from the connection materials, choose a fastener that is cathodic relative to the connection materials.
)
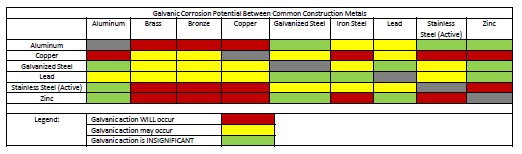
Some good information is available that can help to avoid a galvanic design challenge. First, see Figure 2. This chart provides color-coded galvanic compatibility that is fast and easy to use. The chart suggests material combinations where there will be galvanic action (red), material combination that might demonstrate galvanic activity (yellow), and material combinations that will have insignificant galvanic activity (green).
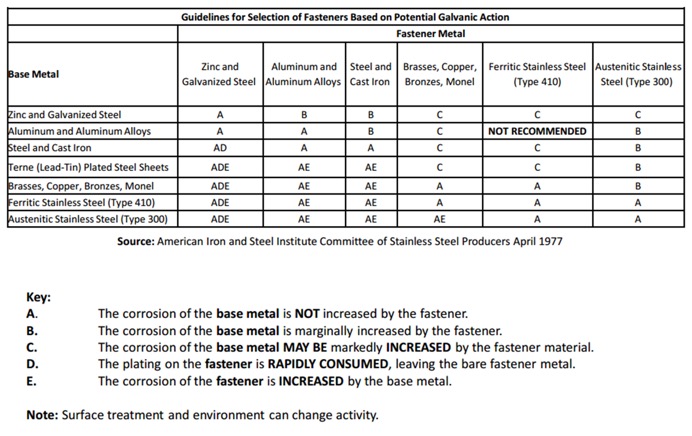
Then see Figure 3 because it gives more information about choices of materials for the fastener and connection materials. Here the probable results of galvanic corrosion to the fastener and base metals are described for various common combinations of common construction materials. It will help to explain which parts of the connection will be affected by galvanic corrosion and how severe the corrosion is likely to be.
We know that you have many challenges when designing fastener connections, and it is our hope that this discussion helps you make informed choices when fastening dissimilar materials. Remember: Galvanic corrosion happens! Let us know if you have any comments.